Parenteral Nutrition
Assess
Page Contents

Gathering Data for An Assessment
Before asking the patient questions for your assessment, make sure to introduce yourself and set the agenda for the discussion.
You may review your patient’s chart or obtain information from them directly. You will need to gather information on the following:
- Clinical Data: past medical history (PMHx), history of presenting illness (HPI), imaging, investigations, pathology, scheduled procedures, consultations, medical orders (medications, infusions), clinical documentation (fluids in and out, bowel movements, drains and tubes, vitals, and documentation of symptoms), medical plan, disposition plan.
- Anthropometric Data: weight, height, BMI, weight change, % weight change, % usual body weight, physical assessment, subjective global assessment.
- Nutritional Requirements: energy, protein, and fluid
- Biochemical Data: laboratory values (blood, urine, feces, sputum, tissue, wounds, drains etc.).
- Dietary Data: current/recent hospital diet order(s), current, recent, and baseline intake, dietary restrictions, allergies/ intolerances, eating behaviours and patterns, calorie counts, supplements, previous nutrition/dietitian interventions.
The components reviewed in each section are common considerations but you may need to consider other factors depending on your patient.
Case Study: Meet Poppy

You are a Registered Dietitian in the Intensive Care Unit (ICU). The patient you are assessing is a 76-year-old female named Poppy.
Assessment Methods
Head-to-toe
The head-to-toe assessment method is used in the intensive care unit. This method could be used in other areas if you encounter an acutely ill or complex patient. You may encounter this terminology when reviewing chart notes of patients who have been transferred from the ICU; therefore it is important to be familiar with it.
| System | Considerations |
|---|---|
| Neurological (CNS) | SAS (sedation agitation scale) score, GCS (Glascow coma scale), Sedation, Paralytics |
| Respiratory (Resp)
|
If vented: type of breathing tube (ETT, type of ), vent settings (FiO2, PEEP, PS/PC, SaO2), respiratory rate (RR), secretions (type and frequency)
If not vented: type of trach, mode of oxygenation (BiPAP, CPAP, Optiflow, face mask, trach mask, nasal prongs (NP)), amount of oxygenation, secretions (type and frequency) |
| Cardiovascular (CVS) | Intra-aortic balloon pump (IABP) and settings, heart rate (HR), blood pressure (BP), mean arterial pressure (MAP), IV access and solution |
| Gastrointestinal (GI) | Abdomen soft vs. firm, distended, type of feeding tube, NG output mode (suction/drainage/clamp) + 24 hour output, feeding formula/rate, amount of daily protein supplementation, nausea/vomiting, bowel movements (type and frequency), 24 hour stoma output, labs (lactate, liver function tests (LFT’s)) |
| Genitourinary (GU) | Urine output (mL/hr, mL/day), 24hr fluid balance, diuretics, dialysis, labs (urea, creatinine, K, PO4, sodium) |
| Infectious Disease | Temperature (max in 24 hr), microbiology result, antibiotics, labs (white blood count (WBC)) |
| Physical Assessment | Generalized/pitting edema, ascites, wounds, lean body mass wasting, |
Subjective Global Assessment
Another important assessment strategy is to physically evaluate your patient for signs and symptoms of malnutrition.
According to the Canadian Malnutrition Task Force:
Subjective global assessment (SGA) is the gold standard for diagnosing malnutrition. SGA is a simple bedside method used to diagnose malnutrition and identify those who would benefit from nutrition care. The assessment includes taking a history of recent intake, weight change, gastrointestinal symptoms and a clinical evaluation.
The SGA provides guidance on how to complete a physical examination by using a head-to-toe method for the assessment of muscle wasting, subcutaneous fat and fluid retention.
| Physical Examination | Normal | Moderate | Severe |
|---|---|---|---|
| Temple | Well-defined muscle | Slight depression | Hollowing, depression |
| Clavicle | Not visible in males, may be visible but not prominent in females | Some protrusion; may not be all the way along | Protruding/prominent bone |
| Shoulder | Rounded | No square look, process may protrude slightly | Square look, bone prominent |
| Scapula/ribs | Bones not prominent | Mild depression or bone may show slightly | Bone prominent, significant depressions |
| Quadriceps | Well defined | Depression/ atrophy medially | Prominent knee, severe depression medially |
| Interosseous muscle between thumb and forefinger (back of hand)** | Muscle protrudes, could be flat in females | Slightly depressed | Flat or depressed area |
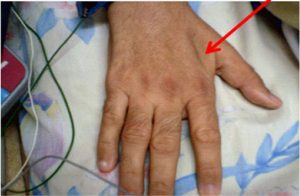
| Physical Examination | Normal | Moderate | Severe |
|---|---|---|---|
| Under the eyes | Slightly bulging area. | Somewhat hollow look, slightly dark circles. | Hollowed look, depression, dark circles. |
| Triceps | Large space between fingers. | Some depth to fat tissues, but not ample. Loose fitting skin. | Very little space between fingers or finger touch. |
| Ribs, lower back, sides of trunk | Chest is full, ribs do not show. Slight to no protrusion of the . | Ribs obvious, but indentations are not marked. Iliac crest somewhat prominent. | Indentation between ribs obvious. Iliac crest very prominent. |
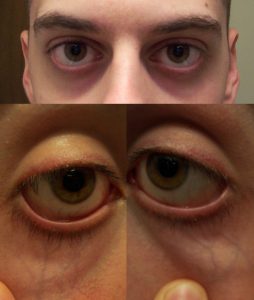
| Physical Examination | Normal | Moderate | Severe |
|---|---|---|---|
| Edema | None | Pitting edema of extremities / pitting to the knees, possible edema if bedridden | Pitting beyond knees, sacral edema if bedridden, may also have generalized edema |
| Ascites | Absent | Present (may only be present on imaging) | Present (may only be present on imaging) |
Physical Assessment
In addition to the head to toe method and the SGA, it is important to go into your patient’s room to evaluate physical signs and symptoms of nutrition deficiencies. It is important to consider evaluating skin integrity, face, mouth, abdomen, temperature and respiration, when possible.
| Site | Physical Examination | Potential Nutritional/ Metabolic Status |
|---|---|---|
| Skin Integrity |
|
|
| Face | Moon face or bilateral temporal wasting | Protein- calorie malnutrition |
| Mouth | Dry, cracked, red lips | Riboflavin, niacin, B12 deficiency |
| Abdomen | Rounded, distended | Gas, edema, ascites, obesity |
| Temperature | Increased temperature | Increased energy and fluid requirements |
| Respiration | Increased respiratory rate | Altered calorie and protein requirements
Energy needs may be increased if weaning from ventilator or decreased if chronically ventilator dependent |
Clinical Data
Clinical data can include, but is not limited to:
- Reason for visit: hospital visit or RD consult.
- Past medical history (PMHx): health history to date.
- History and presenting illness (HPI): symptoms, surgeries, prognosis, tests (i.e. CT scan, ultrasound).
- Current medical orders: IV infusions, medications, consultations.
- Clinical documentation: Fluids intake (i.e. oral, IV, TPN/EN) and output (i.e. urine, vomit, bowel movements, drains (i.e. catheter, chest tube, surgical site drain) and suctioning (i.e. oral secretions, OGT to straight drain), documentation of tubes (i.e. G-tube vs. NGT) and lines (i.e. PICC), vitals.
- Medical care plan and disposition: chemotherapy, radiation therapy, scheduled surgery, transfer to different floor, rehab facility, treatment facility, long term care, home.
Poppy’s Clinical Data
Review Poppy’s clinical data. Take note of components that you think may be important for your parenteral nutrition care plan.
- Age: 76-year-old female
- HPI: chest pain, severe SOB
- Admission: 3 days ago – severe mitral regurgitation
- PMHx: Severe peripheral vascular disease (PVD), abdominal aortic aneurysm (AAA) repair 1991, superior mesenteric artery (SMA) stent 2013
- Operations/ Procedures: OR on day 2 – mitral valve repair (MVR) and coronary artery bypass graft (CABG×2), PICC line inserted today
- Consultations: RD consult for initiation of TPN
- Infusions: IV Normal Saline (NS) @ 10 mL/hr = 240 mL/day, NGT in situ (for drainage of gastric contents and trial of enteral feeds)
- Medications (via triple lumen catheter or NGT):
- Docusate sodium 100 mg
- Sennosides 17.2mg @ 1000 h
- Magnesium Hydroxide 30mL @ 1000 h
- Pantoprazole 40 mg IV @1000 h
- Furosemide 20mg IV BID
- Amiodarone 300 mg IV @ 2200 h
When reviewing medications, you should always have knowledge of what each one is used for and why your patient may be on them even if it does not directly affect your care plan.
- Docusate Sodium: stool softener, used to prevent/treat constipation.
- Sennosides (or Senna): used to treat constipation and empty the large intestine.
- Magnesium Hydroxide: reduces stomach acid and increases water in the intestines which may induce bowel movements. Used as a laxative to relieve constipation or as an antacid.
- Pantoprazole: a proton pump inhibitor used to decrease acid production in the stomach. It helps prevent stress ulcers.
- Furosemide (Lasix): a diuretic used to treat edema and promote urinary fluid loss. It will cause potassium losses in the urine.
- Amiodarone: antiarrhythmic medication used to treat and prevent irregular heartbeats.
Poppy’s Head-to-Toe Assessment Data
Here is an example of the head-to-toe assessment method.
| System | Poppy’s assessment data |
|---|---|
| Neurological (CNS) | Decreased , does not obey, is protecting airway |
| Respiratory (Resp) | Extubated, on 2L NP, RR 18, stating 98% |
| Cardiovascular (CVS) | Hemodynamically stable |
| Gastrointestinal (GI) | Distended abdomen, abdominal pain, dietary order is , NGT in situ |
| Genitourinary (GU) | 1560mL/24hrs urine output, evidence of fluid overload (++), on diuretic (furosemide) |
| Infectious Disease | Afebrile |
| Physical Assessment | Edema in hands and feet, evidence of lean body mass wasting (temporal pitting) |
Poppy has been extubated from the mechanical ventilator but remains in the ICU due to being fluid overloaded and having a low level of consciousness. Consider what this may suggest and how these factors may impact your nutrition care plan.
Anthropometric Data
Assessment of Body Weight
Body weight is the most used indicator of nutritional status, as it is used for calculating fluid, protein, and energy requirements. It is important to consider if the weight you are using needs to be adjusted for fluid retention or if the patient has an amputation. For the most accurate estimations, using a weight as close to a “dry weight” is best.
Obtaining height and age is often necessary to further interpret body weight. Body Mass Index (BMI) is commonly used as a classification to evaluate health risk. However, Master’s tables are used to determine ideal body weight in adults aged 65+.
| Height | Five-Year Age Groups | ||
|---|---|---|---|
| 65–69 years | 70–74 years | 75–79 years | |
| 147 cm | 54.4–66.2 kg | 50.8–62.6 kg | 50.3–61.2 kg |
| 150 cm | 54.9–66.7 kg | 51.7–63.5 kg | 50.8–61.7 kg |
| 152 cm | 55.3–67.1 kg | 52.6–64.4 kg | 51.3–63.1 kg |
| 155 cm | 55.8–68.5 kg | 53.5–65.3 kg | 52.2–64.0 kg |
| 157 cm | 56.7–69.4 kg | 54.9–66.7 kg | 53.5–65.3 kg |
| 160 cm | 57.6–70.3 kg | 55.8–68.5 kg | 54.9–66.7 kg |
It is also important to use other markers of weight, including % weight change and % usual body weight during your assessment to further evaluate your patient’s weight. The calculations in the tables below will help you interpret the findings in regard to severity and indication of malnutrition.
| Time Frame | Significant Weight Loss (%) | Severe Weight Loss (%) |
|---|---|---|
| 1 week | 1-2 | > 2 |
| 1 month | 5 | > 5 |
| 3 months | 7.5 | > 7.5 |
| 6 months | 10 | > 10 |
| Unlimited time | 10-20 | > 20 |
| UBW range (%) | Interpretation |
|---|---|
| 85 – 95 | May indicate mild malnutrition |
| 75 – 84 | May indicate moderate malnutrition |
| < 74 | May indicate severe malnutrition |
Poppy’s Anthropometric Data: Body Weight
- Age: 76 years old
- Height: 160 cm
- Current weight, pre-operative: 63 kg
- Current weight, post-operative: 73 kg (+10 L of fluid)
- Usual weight: 68 kg (1 month prior to admission)
- Timeframe of weight loss: 1 month
- Master’s Table evaluation: within range
- 54.9–66.7 kg is average weight range for women of Poppy’s height and age
- Therefore, Poppy’s pre-operative weight of 63 kg is within range
- However, we still need to consider weight in the context of other factors such as her recent weight loss
- % UBW: 92.6%
- Calculation: (63kg ÷ 68kg) × 100 = 92.6%
- % weight loss: 7.4%
- Calculation: ([68kg − 63kg] ÷ 68kg) × 100 = 7.4%
Nutritional Requirements
Energy Requirements
Predictive equations are for estimation purposes only. The most accurate data will provide the most accurate estimation, but without indirect calorimetry this is the best we can achieve. As a result, there is a need for frequent re-assessment of energy requirements.
Factors affecting the accuracy of estimated requirements include:
- Acute or chronic respiratory distress syndrome
- Large open wounds or burns
- Malnutrition with altered body composition
- Underweight, obesity, limb amputation, peripheral edema, ascites
- Multiple or neurological trauma
- Multisystem organ failure
- Postoperative organ transplantation
- Sepsis
- Systemic inflammatory response syndrome
- Paralytic or barbituate agents
Predictive Equations
Here are three commonly used predictive equations. There are other predictive equations you may use, depending on your area of practice. Accuracy varies by equation and population. Experience is helpful for an accurate selection and utilization of these predictive equations.
Abbreviations:
- EER = estimated energy requirements
- REE = resting energy expenditure (kcal)
- A = age (years)
- PA = physical activity
- W = weight (kilograms)
- H = height (centimetres, unless otherwise specified)
- H* = height (metres)
- Dietary Reference Intakes (DRI)
- EER: age, physical activity, weight, height
- Males: EER (kcal) = 662 − 9.53A + PA × (15.91W + 549.6H*)
- Females: EER (kcal) = 354 − 6.91A + PA × (9.36W + 726H*)
- Harris Benedict (HB)
- REE: weight, height, age
- Males: REE (kcal) = 66.5 + 13.75W + 5.0H − 6.78A
- Females: REE (kcal) = 655.1 + 9.56W + 1.85H − 4.68A
- Mifflin-St.Jeor (MSJ)
- REE: weight, height, age
- Males: REE (kcal) = 9.99W + 6.25H − 4.92A + 5
- Females: REE (kcal) = 9.99W + 6.25H − 4.92A − 161
- Estimated Calories/kg
Specific Predictive Equations for Critically Ill Patients
- BMI 18.5-24.9, vented: 15-40 kcal/kg actual wt (varies based on patient population)
- Obese critically ill, vented: BMI 30-50 = 11-14 kcal/kg actual wt/day; BMI >50 = 22-25 kcal/kg IBW
A second method used for critically ill patients is the Penn-State Equation. Similarly to calculating calories per kilogram, the Penn-State Equation varies by BMI and also patient age (as detailed below).
- Original: Mifflin equation(0.96) + Tmax(167) + Ve(32) − 6212
- Modified for use in patients > 60 years, with BMI >30: Mifflin(0.71) + Tmax(85) + Ve(64) − 3085
- Note: Use actual weight for Mifflin equation; Ve = minute ventilation at time of Tmax
Activity Factors
Most predictive equations evaluate resting energy expenditure (REE), meaning you need to consider physical activity energy expenditure in addition to the original calculation. The activity factor (AF) is applied to the REE value. Activity factors are not to be used with DRI equations.
| Activity Level | AF |
|---|---|
| *Resting (lying or sitting) | 1.0 – 1.4 |
| Lying still, sedated or asleep | 0.9 – 1.1 |
| Lying still, conscious | 1.0 – 1.1 |
| Spinal cord injury, 0-4 weeks post-injury | 1.1 |
| Bedrest (moving self around bed) | 1.15 – 1.2 |
| Mobilizing occasional on ward | 1.15 – 1.4 |
| *Sedentary/ Light Activity (standing for long periods) | 1.4 – 1.6 |
| Mobilizing frequently on ward | 1.4 – 1.5 |
| Regular, intensive physiotherapy | 1.5 – 1.6 |
| *Moderate Activity (continuous movement/slow walking) | 1.6 – 1.8 |
Stress Factors
Most predictive equations evaluate resting energy expenditure (REE), meaning you may need to consider energy expenditure from stress. The stress factor is applied to the REE value.
| Clinical Status | SF |
|---|---|
| Cancer | 0.8 – 1.5 |
| Elective surgery | 1.0 – 1.1 |
| Peritonitis | 1.05 – 1.25 |
| Multiple/ long bone fractures | 1.1 – 1.3 |
| Fever | 1.2 per 1°C > 37°C |
| Spinal cord injury, 0-4 weeks post-injury | 1.2 |
| Sepsis | 1.2 – 1.4 |
| Severe infection | 1.2 – 1.6 |
| Burns | 1.2 – 2.0 |
| Infection with trauma | 1.3 – 1.55 |
| Multiple trauma, traumatic brain injury | 1.4 |
Protein Requirements
This table represents general guidelines for protein requirements according to how hypermetabolic your patient is. Consider your individualized patient to determine the most accurate protein requirement. Experience using these methods is helpful. You can consider calculating requirements using various methods and compare values.
| Patient Category | Protein (g/kg) |
|---|---|
Not hypermetabolic:
|
0.8 – 1.5
(1.0 – 1.5 for |
Moderately hypermetabolic, including:
|
1.2 – 1.5 |
| Hypermetabolic, including multi-trauma |
1.5 – 2.0
|
The following table represents a more detailed overview of protein requirements specific to various clinical conditions. This may be more useful for you during practice than the general guidelines, if your patient’s clinical status is reflected in this table.
| Clinical Status | Protein (g/kg) |
|---|---|
| Normal (non-stressed, non-depleted) | 0.8 – 1.0 |
| Postoperative | 1.0 – 1.5 |
| Sepsis | 1.5 – 2.0 |
| Multiple trauma | 1.3 – 1.7 |
| Traumatic brain injury | 1.2 – 2.0 |
| Burns | 1.2 – 2.0 |
| Catabolism | 1.2 – 2.0 |
| Refeeding syndrome | 1.2 – 1.5 |
| Cancer | 0.8 – 2.0 |
| Hemodialysis | 1.1 – 1.2 |
| CCPD/CAPD | 1.2 – 1.3 |
| CRRT | 1.5 – 2.0 |
| Acute Renal Failure | 1.0 – 1.5 |
| Chronic Kidney Disease | 0.8 – 1.0 |
| Mild-Moderate Stress | 1.2 – 1.3 |
| Moderate-Severe Stress | 1.5 – 2.0 |
| Severe + Wound Healing | 1.5 – 2.0 |
| HIV (asymptomatic) | 1.0 – 1.4 |
| HIV (symptomatic) | 1.5 – 2.0 |
| HIV (CD4 < 200/AIDS defining condition) | 2.0 – 2.5 |
Fluid Requirements
This table represents general guidelines for calculating fluid requirements. Consider your individual patient prior to determining the best method to use. Experience using these methods is helpful. You can calculate requirements using various methods and compare values.
| Based Upon | Method of Fluid Estimation |
|---|---|
| Weight |
|
| Energy | 1 mL per kcal |
| Age and weight |
|
| Fluid balance | Urine output + 500 mL/day |
Electrolyte Requirements
This table provides an example of general guidelines for electrolyte requirements. Be sure to take your patient’s current bloodwork and clinical status into consideration prior to determining their electrolyte requirements. Electrolyte requirements can vary tremendously based on the clinical situation.
| Electrolyte | Daily requirements | Factors that increase needs |
|---|---|---|
| Sodium | 1 – 2 mmol/kg | Diarrhea, vomiting, GI losses |
| Potassium | 1 – 2 mmol/kg | Diarrhea, vomiting, medications, refeeding syndrome, GI losses |
| Calcium | 5 – 7.5 mmol/day | High protein intake |
| Magnesium | 4 – 10 mmol/day | Medications, refeeding syndrome, GI losses |
| Phosphorous | 20 – 40 mmol/day | High dextrose loads, refeeding syndrome |
Biochemical Data
Laboratory Values
| Laboratory Value | Normal Range |
|---|---|
| WBC | 4.00 – 11.00 E9/L |
| Glucose (Random) | 4.0 – 7.8 mmol/L |
| Sodium (Na+) | 135 – 145 mmol/L |
| Potassium (K+) | 3.5 – 5.0 mmol/L |
| Chloride (Cl-) | 96 – 106 mmol/L |
| Phosphorus (PO4) | 0.8 – 1.35 mmol/L |
| Calcium (Ca2+) | 2.1 – 2.1 mmol/L |
| Magnesium (Mg2+) | 0.63 – 0.94 mmol/L |
| Albumin (Alb) | 35 – 50 g/L |
| Blood Urea Nitrogen (BUN) | 3.0 – 7.0 mmol/L |
| Creatinine (Cr) | 44 – 80 μmol/L |
Poppy’s Biochemical Data
Review Poppy’s biochemical data and make note of values highlighted in red.
| Laboratory Value | Poppy’s labs |
|---|---|
| White blood cell count (wbc) | *14.7 109/L |
| Hemoglobin (Hgb) | *95 g/L |
| Glucose (BG) | 6.2 mmol/L |
| Sodium (Na+) | *132 mmol/L |
| Potassium (K+) | 4.9 mmol/L |
| Chloride (Cl–) | 107 mmol/L |
| Phosphorus (PO4) | 1.33 mmol/L |
| Calcium (Ca2+) | 2.20 mmol/L |
| Magnesium (Mg2+) | *1.0 mmol/L |
| Albumin (Alb) | *25 g/L |
| Blood Urea Nitrogen (BUN) | *9.8 mmol/L |
| Creatinine (Cr) | *121 μmol/L |
Here is a general assessment of Poppy’s biochemical data. It is important to understand why values are not in the normal range in the context of the patients clinical status.
- WBC: Elevated, may indicate development of an infection or tissue damage. An infection may further increase Poppy’s metabolic rate.
- Hemoglobin: Low, likely multifactorial: blood loss during surgery, bone marrow suppression due to renal failure and malnutrition. Her history does not suggest she is actively bleeding anywhere.
- Sodium: Low, likely hypervolemic hyponatremia due to fluid retention causing dilution of sodium in the blood. It is not low due to sodium loss – but instead it is reflecting her fluid status (i.e. overloaded).
- Glucose: Within normal limits for ICU. Insulin infusion may be started if glycemic control is inadequate.
- BUN: Elevated due to acute kidney injury (AKI)/renal failure.
- Creatinine: Elevated due to AKI/renal failure. Note that the creatinine level has almost doubled over her baseline level in a matter of days. This is an indication of acute kidney injury due to decreased perfusion of blood to the kidneys during cardiovascular surgery.
Poppy’s Nutrition Requirements
- Consideration for requirements: post op cardiovascular surgery, critical illness, sepsis, AKI, catabolic
- Use preoperative weight 63 kg due to current fluid overload (+ 10 L)
- Caloric requirements (calories/kg): 25 – 30 kcal/kg × 63 kg = 1575 – 1890 kcal/day
- Poppy’s Energy Requirements: 1575 – 1890 kcal/day (25 – 30 kcal/kg/day)
- Protein requirements: range from 1.2 – 1.5 g/kg (you should look up factors to be aware of for the assessment of AKI).
- Poppy’s Protein Requirements: 76 – 95 g/day (1.2 – 1.5 g/kg)
- Fluid requirements: 25 – 30 mL/kg x 63 kg = 1575 – 1890 mL/day
- Poppy’s Fluid Requirements: 1575 mL/day (25 mL/kg) – as conservative as possible
- Consideration for Fluid: MD orders (did they specify a fluid target?), ongoing diuresis? As mentioned prior, limiting fluid is a priority.
When calculating energy requirements, Poppy’s preoperative weight will be used. Calories per kilogram is commonly used in the ICU and other areas of practice. It requires clinical judgement of the patient’s status; however you can always calculate energy requirements using multiple methods to compare when you are learning to use these equations. Considerations for Poppy’s energy requirements are that she is post operative and experiencing critical illness.
Poppy’s protein requirements should range from 1.0-1.5 g/kg as she is post operative and experiencing acute kidney injury (AKI). Fluid needs to be minimized as much as possible.
Negotiate a fluid allowance with the Intensivist and/or Nephrologist. Sometimes due to the condition of the patient, they may only want 1 L total fluid per day. A positive fluid balance of 10 L is very high. The electrolytes that need to be addressed are: sodium, potassium, calcium, magnesium and phosphorus.
The initial parenteral nutrition solution may need to be electrolyte free because Poppy’s electrolytes are elevated. Liaise with the pharmacist/intensivist/nephrologist to avoid her blood levels from becoming dangerously elevated.
IV Solutions
Here is an overview of common IV solutions used in hospital. It is important to review which IV solution(s) your patient is receiving as it could be providing a patient with energy/ dextrose. Different IV solutions are also chosen in various clinical scenarios. This needs to be considered when you are creating a nutrition care plan.
| Solution | Kcal/L | Composition/L |
|---|---|---|
| Normal Saline (0.9% NaCl) | 0 | Na – 154 mmol
Cl – 154 mmol |
| ½ Normal Saline (0.45% NaCl) | 0 | Na -77 mmol
Cl – 77 mmol |
| D5W (5% Dextrose) | 170 | Dextrose – 50 g |
| D10W (10% Dextrose) | 340 | Dextrose – 100 g |
| 5% Dextrose and 0.9% NaCl | 170 | Dextrose – 50 g
Na – 154 mmol Cl – 154 mmol |
| ⅔ and ⅓ (3.3% Dextrose and 0.3% NaCl) | 112 | Dextrose – 33 g
Na – 51 mmol Cl – 51 mmol |
| Ringer’s Lactate | 9 | Na – 130 mmol
K – 4 mmol Ca2+ – 1.4 mmol Cl – 109 mmol Lactate – 28 mmol |
Poppy’s IV Solution
Poppy is currently receiving IV NS @ 10cc/hr = 240cc/d. Normal saline does not contribute any calories for Poppy. However it does contribute a small amount of Na (37 mmol) and Cl (37 mmol).
Dietary Data
Obtaining accurate dietary data can vary based on your patient (e.g. family members present, patient’s cognitive ability, flow sheets or calorie counts, etc.), as well as the setting (e.g. inpatient compared to outpatient).
If possible, collect the following information:
- Diet order(s): Important for a representation of daily intake while in the hospital (can include enteral nutrition & supplements).
- Dietary recall: 24 hour recall (if recent admission or representation of food consumption in hospital), common eating patterns, or short-term and long-term representation of eating patterns or typical foods.
- Calorie counts: Depending on the patient, you may order calorie counts to monitor/determine how much/what they are eating in hospital.
Poppy’s Dietary Data
Prior to hospital admission she had been living with her sister, Esther, in an apartment. Poppy was starting to have difficulty mobilizing due to her shortness of breath. She had not been eating well for weeks and had lost ~5 kg of weight during that time frame. Esther summarizes Poppy’s usual daily intake for the past few weeks.
Dietary Recall for Poppy
- Breakfast: 1 cup of tea with milk, 1 piece of whole grain toast with butter and jam
- Lunch: 1 bottle of Ensure
- Afternoon snack: ½ banana, 1 cup of tea with milk
- Dinner: 1 small chicken breast, ½ cup potato, ½ cup vegetables, 1 cup of tea with milk
Energy intake ~965 kcal/day and protein intake ~45 g/day. Poppy is not consuming an adequate amount for her height, weight and age. Her suboptimal oral intake is contributing to her weight loss.
Because Poppy’s diet history is nutritionally inadequate and suggests suboptimal preoperative nutritional status, it is important to note that this can affect her postoperative outcome.
Poppy’s Current Dietary Order and Plan
The Intensivist requested that Poppy start enteral feeding as there is good evidence to support early feeding in ICU patients.
Enteral order, as per the ICU enteral feeding protocol: Isosource 1.5 @ 10 mL/hr × 24 hrs/day via NGT. This is considered a “trickle feed” as it does not provide extensive nutrition but is used to stimulate the gut.
Complications: Within 8 hours Poppy started complaining of diffuse abdominal pain and her nurse noticed increased abdominal distension. Her enteral feeds are held, and a computed tomography (CT) of the abdomen is ordered.
CT Findings: Contrast-enhanced transverse CT scan shows ischemia of the distal ileum, with pronounced bowel wall thickening and mesenteric fat stranding. No bowel obstruction.
Outcome/ Assessment: The Intensivist does not want Poppy fed enterally, so you are asked to assess for TPN. The physicians are concerned about her fluid status as it will negatively impact her respiratory status. Important: They want you to limit fluid in your nutrition care plan.
Practice your Enteral Feeding Skills
Recall that Poppy received Isosource 1.5 @ 10 mL/hr for 8 hours, before her enteral feeds were held.
The table below contains values taken from the Isosource 1.5 nutrition panel.
| Energy | Protein | Fat | Carbohydrate | Water | Sodium | Potassium | Chlorine | Osmolality | |
|---|---|---|---|---|---|---|---|---|---|
| Unit | Kcal/mL | g/mL | g/mL | g/mL | mL | mg | mg/mmol | mg | mOsm/kg H2O |
| Amount (per mL) |
1.5 | 0.068 | 0.06 | 0.17 | 0.76 | 1.3 | 2.4/0.06 | 1.6 | 530 |
Refeeding Syndrome
Refeeding syndrome is a concern for any patient who has been without consistent or adequate nutrition for a prolonged period. It is a series of metabolic events that occur as a result of reinstitution of nutrition (carbohydrates) to patients who are starved or severely malnourished. Refeeding syndrome is characterized by low potassium, magnesium, and phosphate with/or without fluid retention.
Serious complications can be avoided by:
- Thorough nutritional assessment
- Appropriate identification of patients at risk
- Slow initiation of feeding
- Careful monitoring
The table below outlines complications of refeeding syndrome.
| Hypophosphatemia | Hypokalemia | Hypomagnesemia | |
|---|---|---|---|
| Cardiac | Arrhythmia, CHF, cardiomyopathy, decreased blood pressure | Arrhythmia, cardiac arrest, EKG changes | Arrhythmia, increased heart hate |
| Neurological | Altered mental status, paralysis, seizures | Weakness, paralysis, lethargy/ confusion | Altered LOC, weakness, seizures, tremors |
| Respiratory | Respiratory failure, ventilator dependence | N/A | N/A |
| Skeletal | Rhabdomyolysis, weakness | N/A | N/A |
| Metabolomic | N/A | Metabolic alkalosis | Hypokalemia, hypocalcemia |
| Gastrointestinal | N/A | Paralytic ileus, constipation | Abdominal pain, diarrhea, constipation, anorexia |
An additional refeeding complication is hyponatremia secondary to hyperglycemia, which can result in: heart failure, arrhythmia, respiratory failure, pulmonary edema, renal failure, muscle cramps, fatigue, fluid retention, swelling/edema.
Risk factors for refeeding syndrome include:
- Suboptimal or no nutritional intake for > 5 days
- Postoperative
- Elderly with multiple comorbidities and decreased physiological reserve
- Cancer diagnosis
- Chronically malnourished: anorexia nervosa, chronic alcoholism, marasmus, prolonged fasting or low energy diet, morbid obesity with profound weight loss, malabsorptive syndrome (i.e. IBS, chronic pancreatitis, short bowel syndrome), high electrolyte losses (i.e. diarrhea, high output fistula, vomiting)
- Uncontrolled diabetes (i.e. electrolyte depletion, diuresis)
- Long term use of antacids
- Long term use of diuretics
- BMI <18.5
- Ongoing unintentional weight loss
Poppy’s Risk of Refeeding Syndrome
- Poppy is at low- moderate risk of refeeding syndrome.
- Although she has lost some weight preoperatively, she is not cachectic, and was eating a moderate amount of food prior to her surgery.
- She has gone without nutrition support for 3 days. If she does demonstrate refeeding syndrome it may present late due to her AKI/renal failure. Monitor her blood work for electrolyte shifts particularly phosphorous, potassium, magnesium and blood glucose.
Assessment Summary
Summary of Poppy’s Assessment Data
Review the final summary for Poppy’s assessment data. In practice, it is good to have a summary of this information with you at all times and to keep track of the progression of your patient.
| Area | Key Data |
|---|---|
| Clinical Data |
|
| Medications and Infusions |
|
| Head to Toe |
|
| Anthropometrics |
|
| Requirements |
|
| Laboratory |
|
| Dietary |
|
PART 1: ASSESS COMPLETE. Please take a few minutes to think about the assessment strategies discussed and the data collected. When you’re ready, move on to Part 2: Plan.
tracheostomy
excess perspiration/sweating
a bone at the top edge of the shoulder blade
The top of the pelvic bone, at the hip
The sacrum is a large triangular bone at the base of the spine, between the hip bones and above the tailbone.
Essential fatty acid
Common medical abbreviation of "bis in die", Latin for "twice per day".
level of consciousness
Common medical abbreviation for Latin "nil per os", meaning "nothing by mouth".

